UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Charter)
|
| |||
(State or Other Jurisdiction of Incorporation) |
| (Commission File Number) |
| (IRS Employer Identification No.) |
| ||
(Address of Principal Executive Offices) |
| (Zip Code) |
Registrant’s telephone number, including area code: (
Not applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading symbol(s) |
| Name of each exchange on which registered |
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 2.02 Results of Operations and Financial Condition.
On August 14, 2023, Xilio Therapeutics, Inc. (the “Company”) announced its financial results for the quarter ended June 30, 2023 and other business highlights. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein.
Item 7.01 Regulation FD Disclosure.
From time to time, the Company presents or distributes slide presentations to the investment community to provide updates and summaries of its business. The Company is posting a copy of its current corporate investor presentation to the “Investors & Media” portion of its website at https://ir.xiliotx.com. The information contained on, or accessible through, the Company’s website is not incorporated by reference into this Current Report on Form 8-K and should not be considered to be a part hereof. A copy of the presentation is furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Current Report on Form 8-K, including Exhibits 99.1 and 99.2, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
The following exhibit relating to Item 2.02 and Item 7.01 of this Current Report on Form 8-K shall be deemed to be furnished and not filed:
Exhibit No. |
| Description |
99.1 |
| Press release issued by Xilio Therapeutics, Inc. on August 14, 2023 |
99.2 | Corporate investor presentation of Xilio Therapeutics, Inc. as of August 14, 2023 | |
104 |
| Cover Page Interactive Data File (embedded within the Inline XBRL document and incorporated as Exhibit 101) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
XILIO THERAPEUTICS, INC. | ||
|
|
|
Date: August 14, 2023 | By: | /s/ René Russo |
|
| René Russo |
|
| Chief Executive Officer |
Exhibit 99.1
Xilio Therapeutics Announces Pipeline and Business Updates and Second Quarter 2023 Financial Results
Xilio entered into clinical trial collaboration with Roche to evaluate XTX101, a tumor-activated, Fc-enhanced anti-CTLA-4, in combination with atezolizumab (Tecentriq®), in patients with microsatellite stable (MSS) colorectal cancer
Xilio to host live virtual program spotlighting XTX101 on Thursday, August 17, 2023, at 12:30 p.m. ET
Anticipate reporting preliminary Phase 1/2 data for XTX202, a tumor-activated IL-2, in early November 2023
Anticipate reporting preliminary Phase 1 safety data for XTX301, a tumor-activated IL-12, in the fourth quarter of 2023
Ended second quarter of 2023 with $75.4 million in cash and cash equivalents, with cash runway anticipated into the end of the second quarter of 2024
WALTHAM, Mass., August 14, 2023 – Xilio Therapeutics, Inc. (Nasdaq: XLO), a clinical-stage biotechnology company discovering and developing tumor-activated immuno-oncology therapies for people living with cancer, today announced pipeline progress and business updates and reported financial results for the second quarter ended June 30, 2023.
“In the second quarter, we reported encouraging initial monotherapy data from our Phase 1 trial of XTX101, a tumor-activated, Fc-enhanced anti-CTLA-4, including a partial response in a patient with advanced non-small cell lung cancer and a favorable preliminary safety profile at the recommended Phase 2 dose,” said René Russo, Pharm.D., chief executive officer of Xilio. “Building on these data, today we are pleased to announce a clinical trial collaboration with Roche to evaluate XTX101 in combination with atezolizumab in patients with MSS colorectal cancer. Currently, there are no approved immunotherapies for patients living with this cold tumor type. We believe the combination of our tumor-activated anti-CTLA-4 with an anti-PD-L1 represents a promising combination for evaluation in MSS colorectal cancer and potentially other cold tumor types with limited treatment options. We also look forward to reporting preliminary clinical data later this year from our ongoing trials of XTX202, a tumor-activated IL-2, and XTX301, a tumor-activated IL-12.”
Pipeline and Business Updates
XTX101: tumor-activated, Fc-enhanced anti-CTLA-4
XTX101 is an investigational tumor-activated, Fc-enhanced, high affinity binding anti-CTLA-4 designed to block CTLA-4 and deplete regulatory T cells when activated (unmasked) in the tumor microenvironment (TME). XTX101 has completed monotherapy dose escalation (Part 1A) and is currently being evaluated at the recommended Phase 2 dose and schedule of 150 mg once every six weeks (RP2D) in monotherapy dose expansion (Part 1B) of an ongoing Phase 1 clinical trial in patients with advanced solid tumors.
· | Xilio today announced that it has entered into a clinical trial collaboration with Roche to evaluate XTX101 in combination with atezolizumab (Tecentriq®) in a Phase 1/2 clinical trial consisting of Phase 1 combination dose escalation in patients with advanced solid tumors and Phase 2 in patients with microsatellite stable colorectal cancer (MSS CRC). Under the clinical trial supply agreement, Xilio is eligible to receive specified cost-sharing payments from Roche, and each |
company will supply its respective anti-cancer agent to support the clinical trial. Xilio will sponsor and conduct the Phase 1/2 clinical trial and retains global development and commercialization rights to XTX101.
· | In May 2023, Xilio announced preliminary monotherapy data from its Phase 1 clinical trial evaluating XTX101 in patients with advanced solid tumors. These data included a confirmed partial response observed in a patient with advanced non-small cell lung cancer and a favorable preliminary safety profile observed at the RP2D. For more information, read the press release here. |
· | Xilio today announced updated monotherapy data from its Phase 1 clinical trial evaluating XTX101 in patients with advanced solid tumors at the RP2D. As of a data cutoff date of August 3, 2023, 11 patients had been treated at the RP2D. Across all dosing levels and dosing intervals, no Grade 4 or Grade 5 treatment-related adverse events (AEs) were reported by investigators. Among the 9 patients who received XTX101 administered at the RP2D and for whom safety data were available as of the data cutoff date, the most common treatment-related AEs (≥10% incidence) of any grade reported by investigators were diarrhea (11%), fatigue (11%), decreased appetite (11%) and dermatitis (11%). In these patients, no treatment-related colitis or infusion related reaction of any grade was observed. In addition to a previously reported Grade 3 treatment-related AE of diarrhea, which resolved after five days without steroid use, investigators observed one Grade 3 treatment-related AE of dermatitis. As of the data cutoff date of August 3, 2023, no patients who received XTX101 administered at the RP2D have discontinued treatment due to a treatment-related AE. In addition, Xilio reported data showing a durable, continuing partial response of 36 weeks in the previously reported patient with advanced non-small cell lung cancer, with treatment ongoing as of the data cutoff date. |
Xilio anticipates activating clinical trial sites for the Phase 1 dose escalation portion of the clinical trial evaluating XTX101 in combination with atezolizumab in the fourth quarter of 2023.
XTX202: tumor-activated, engineered, beta-gamma biased IL-2
XTX202 is an investigational tumor-activated beta-gamma biased, engineered IL-2 molecule designed to potently stimulate CD8+ effector T cells and natural killer (NK) cells without concomitant stimulation of regulatory T cells when activated (unmasked) in the TME. XTX202 is currently being evaluated in an ongoing Phase 1/2 clinical trial in patients with advanced solid tumors.
· | Xilio recently cleared the 2.8 mg/kg dose level (dose level six) in monotherapy dose escalation for the Phase 1 clinical trial. No signs or symptoms of vascular leak syndrome have been observed in patients through the 2.8 mg/kg dose level. |
· | Xilio is currently dosing patients at the 4.0 mg/kg dose level (dose level seven) in monotherapy dose escalation for the Phase 1 clinical trial. A maximum tolerated dose has not yet been determined, and enrollment in the Phase 1 clinical trial is ongoing. |
· | In addition, Xilio continues to dose patients at the 1.4 mg/kg dose level in the Phase 2 clinical trial evaluating XTX202 as a monotherapy in patients with unresectable or metastatic melanoma and metastatic renal cell carcinoma who have progressed on standard-of-care treatment. Based on continued Phase 1 monotherapy dose escalation, Xilio also plans to explore opportunities to evaluate XTX202 at an additional, higher dose level in the Phase 2 clinical trial. |
Xilio anticipates reporting preliminary anti-tumor activity, safety, pharmacokinetic and pharmacodynamic data from the Phase 1/2 clinical trial in early November 2023. The company anticipates the reported data will include at least 20 evaluable patients across a range of solid tumors treated at dose levels of 1.0 mg/kg or higher across all cohorts in the Phase 1/2 clinical trial.
In addition, Xilio today announced the acceptance of an abstract for XTX202 at the Society for Immunotherapy of Cancer (SITC) 38th Annual Meeting on November 1-5, 2023.
XTX301: tumor-activated, engineered IL-12
XTX301 is an investigational tumor-activated, engineered IL-12 molecule designed to potently stimulate anti-tumor immunity and reprogram the TME of poorly immunogenic “cold” tumors towards an inflamed or “hot” state.
· | Xilio is currently dosing patients in monotherapy dose escalation of an ongoing Phase 1 clinical trial evaluating the safety and tolerability of XTX301 in patients with advanced solid tumors. |
· | Xilio anticipates reporting preliminary safety data from the Phase 1 clinical trial into the third dose level in the fourth quarter of 2023. |
Corporate Highlights
· | In August 2023, Xilio announced the promotion of Chris Frankenfield to Chief Operating Officer. |
Virtual Spotlight Program on XTX101
Xilio will host a live virtual program on Thursday, August 17, 2023, at 12:30 p.m. ET spotlighting XTX101, including highlights from the recently reported Phase 1 monotherapy data for XTX101 and clinical development plans in MSS CRC.
· | The event will feature members of Xilio’s management team as well as Dr. Diwakar Davar, MBBS, M.Sc., a key opinion leader and assistant professor of medicine and a medical oncologist/hematologist from UPMC Hillman Cancer Center. Dr. Davar will discuss the anti-CTLA-4 treatment landscape, including recent advances observed in patients with previously I-O refractory cold tumors, such as MSS CRC. A live question and answer session will follow the presentation. |
· | To register in advance for the webcast, please click here. A live webcast of the event will also be available under “Events and Presentations” in the Investors & Media section of Xilio’s website at https://ir.xiliotx.com. A replay of the webcast will be archived on Xilio’s website for 90 days following the event. |
Second Quarter 2023 Financial Results
· | Cash Position: Cash and cash equivalents were $75.4 million as of June 30, 2023, compared to $120.4 million as of December 31, 2022. |
· | Research & Development (R&D) Expenses: R&D expenses were $13.2 million for the quarter ended June 30, 2023, compared to $16.2 million for the quarter ended June 30, 2022. The decrease was primarily driven by decreased manufacturing and clinical development activities for XTX101 and decreased manufacturing and preclinical activities for XTX301. These decreases were partially offset by increases in clinical activities for XTX202 and XTX301. |
· | General & Administrative (G&A) Expenses: G&A expenses were $6.9 million for the quarter ended June 30, 2023, compared to $8.3 million for the quarter ended June 30, 2022. The decrease was primarily driven by a decrease in personnel-related costs, including stock-based compensation. |
· | Net Loss: Net loss was $19.4 million for the quarter ended June 30, 2023, compared to $24.6 million for the quarter ended June 30, 2022. |
Financial Guidance
Xilio continues to anticipate that its existing cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements into the end of the second quarter of 2024.
About XTX101 (anti-CTLA-4) and the Phase 1 Monotherapy and Phase 1/2 Combination Clinical Trials
XTX101 is an investigational tumor-activated, Fc-enhanced, high affinity binding anti-CTLA-4 monoclonal antibody designed to block CTLA-4 and deplete regulatory T cells when activated (unmasked) in the tumor microenvironment (TME). The Phase 1 clinical trial is a first-in-human, multi-center, open-label trial designed to evaluate the safety and tolerability of XTX101 for the treatment of adult patients with advanced solid tumors. Xilio has completed monotherapy dose escalation (Part 1A) and is currently enrolling patients at the recommended Phase 2 dose and schedule of 150 mg once every six weeks in monotherapy dose expansion (Part 1B). Please refer to NCT04896697 on www.clinicaltrials.gov for additional details.
In addition, Xilio plans to evaluate the safety, tolerability and efficacy of XTX101 in combination with atezolizumab (Tecentriq®) in the Phase 1/2 clinical trial. The Phase 1 portion is designed to assess the safety and tolerability of XTX101 in combination with atezolizumab in dose escalation in patients with advanced solid tumors. The planned Phase 2 portion is designed to evaluate the safety and efficacy of the combination in patients with microsatellite stable colorectal cancer (MSS CRC).
About XTX202 (IL-2) and the Phase 1/2 Clinical Trials
XTX202 is an investigational tumor-activated beta-gamma biased, engineered IL-2 molecule designed to potently stimulate CD8+ effector T cells and natural killer (NK) cells without concomitant stimulation of regulatory T cells when activated (unmasked) in the tumor microenvironment (TME). The Phase 1 clinical trial for XTX202 is a first-in-human, multi-center, open-label trial designed to evaluate the safety and tolerability of XTX202 as a monotherapy in patients with advanced solid tumors. The Phase 2 clinical trial for XTX202 is a multi-center, open-label trial designed to evaluate the safety and efficacy of XTX202 as a monotherapy in patients with unresectable or metastatic melanoma and metastatic renal cell carcinoma who have progressed on standard-of-care treatment. Please refer to NCT05052268 on www.clinicaltrials.gov for additional details.
About XTX301 (IL-12) and the Phase 1 Clinical Trial
XTX301 is an investigational tumor-activated, engineered IL-12 molecule designed to potently stimulate anti-tumor immunity and reprogram the tumor microenvironment (TME) of poorly immunogenic “cold” tumors towards an inflamed or “hot” state. The Phase 1 clinical trial for XTX301 is a first-in-human, multi-center, open-label trial designed to evaluate the safety and tolerability of XTX301 as a monotherapy in patients with advanced solid tumors. Please refer to NCT05684965 on www.clinicaltrials.gov for additional details.
About Xilio Therapeutics
Xilio Therapeutics is a clinical-stage biotechnology company discovering and developing tumor-activated immuno-oncology (I-O) therapies with the goal of significantly improving outcomes for people living with cancer without the systemic side effects of current I-O treatments. The company is using its proprietary geographically precise solutions (GPS) platform to build a pipeline of novel, tumor-activated molecules, including antibodies, cytokines and other biologics, which are designed to optimize their
therapeutic index and localize anti-tumor activity within the tumor microenvironment. Xilio is currently advancing multiple programs for tumor-activated I-O treatments in clinical development, as well as programs in preclinical development. Learn more by visiting www.xiliotx.com and follow us on Twitter (@xiliotx) and LinkedIn (Xilio Therapeutics, Inc.).
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding plans, timing and expectations related to reporting preliminary data from the Phase 1/2 clinical trial for XTX202, including the anticipated number of patients treated at the 1 mg/kg dose level or higher; evaluating XTX202 at a second dose level in the Phase 2 clinical trial; reporting preliminary safety data from the Phase 1 clinical trial for XTX301; activating clinical trial sites for Phase 1 combination dose escalation portion of the clinical trial evaluating XTX101 in combination with atezolizumab in patients with advanced solid tumors; the potential benefits of any of Xilio’s current or future product candidates in treating patients; Xilio’s ability to obtain and maintain sufficient cash resources to fund its operations beyond the end of the second quarter of 2024; and Xilio’s strategy, goals and anticipated financial performance, milestones, business plans and focus. The words “aim,” “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “seek,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of important risks, uncertainties and other factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, risks and uncertainties related to ongoing and planned research and development activities, including initiating, conducting or completing preclinical studies and clinical trials and the timing and results of such preclinical studies or clinical trials; the delay of any current or planned preclinical studies or clinical trials or the development of Xilio’s current or future product candidates; Xilio’s ability to obtain and maintain sufficient preclinical and clinical supply of current or future product candidates; Xilio’s advancement of multiple early-stage programs; there can be no assurance that interim or preliminary preclinical or clinical data or results will be predictive of future preclinical or clinical data or results; Xilio’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; results from preclinical studies or clinical trials for Xilio’s product candidates, which may not support further development of such product candidates; actions of regulatory agencies, which may affect the initiation, timing and progress of current or future clinical trials; Xilio’s ability to obtain, maintain and enforce patent and other intellectual property protection for current or future product candidates; Xilio’s ability to obtain and maintain sufficient cash resources to fund its operations beyond the end of the second quarter of 2024; the impact of international trade policies on Xilio’s business, including U.S. and China trade policies; and Xilio’s ability to maintain its clinical trial collaboration with Roche to develop XTX101 in combination with atezolizumab. These and other risks and uncertainties are described in greater detail in the sections entitled “Risk Factor Summary” and “Risk Factors” in Xilio’s filings with the U.S. Securities and Exchange Commission (SEC), including Xilio’s most recent Quarterly Report on Form 10-Q and any other filings that Xilio has made or may make with the SEC in the future. Any forward-looking statements contained in this press release represent Xilio’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Xilio explicitly disclaims any obligation to update any forward-looking statements.
This press release contains hyperlinks to information that is not deemed to be incorporated by reference in this press release.
TECENTRIQ is a registered trademark of Genentech USA, Inc., a member of the Roche Group.
For Investor and Media Inquiries:
Julissa Viana
Vice President,
Head of Investor Relations and Corporate Communications
investors@xiliotx.com
Melissa Forst
Argot Partners
Xilio@argotpartners.com
XILIO THERAPEUTICS, INC.
Condensed Consolidated Balance Sheets
(In thousands)
(Unaudited)
|
| June 30, |
| December 31, | ||
| | 2023 | | 2022 | ||
Assets |
| |
|
| |
|
Cash and cash equivalents | | $ | 75,383 | | $ | 120,385 |
Other assets | |
| 16,976 | |
| 18,780 |
Total assets | | $ | 92,359 | | $ | 139,165 |
Liabilities and Stockholders’ Equity | |
|
| |
|
|
Liabilities | | $ | 24,982 | | $ | 33,518 |
Stockholders’ equity | |
| 67,377 | |
| 105,647 |
Total liabilities and stockholders’ equity | | $ | 92,359 | | $ | 139,165 |
XILIO THERAPEUTICS, INC.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share data)
(Unaudited)
| | Three Months Ended | | Six Months Ended | ||||||||
| | June 30, | | June 30, | ||||||||
|
| 2023 |
| 2022 |
| 2023 |
| 2022 | ||||
Operating expenses (1) |
| |
|
| |
|
| |
|
| |
|
Research and development | | $ | 13,218 | | $ | 16,246 | | $ | 29,349 | | $ | 31,166 |
General and administrative | |
| 6,898 | |
| 8,306 | |
| 14,293 | |
| 14,610 |
Total operating expenses | |
| 20,116 | |
| 24,552 | |
| 43,642 | |
| 45,776 |
Loss from operations | |
| (20,116) | |
| (24,552) | |
| (43,642) | |
| (45,776) |
Other income (expense), net | |
|
| |
|
| |
|
| |
|
|
Other income (expense), net | |
| 761 | |
| (61) | |
| 1,641 | |
| (190) |
Total other income (expense), net | |
| 761 | |
| (61) | |
| 1,641 | |
| (190) |
Net loss and comprehensive loss | | $ | (19,355) | | $ | (24,613) | | $ | (42,001) | | $ | (45,966) |
Net loss per share, basic and diluted | | $ | (0.70) | | $ | (0.90) | | $ | (1.53) | | $ | (1.68) |
Weighted average common shares outstanding, basic and diluted | |
| 27,468,668 | |
| 27,384,614 | |
| 27,451,058 | |
| 27,376,043 |
(1) | Operating expenses include the following amounts of non-cash stock-based compensation expense: |
| | Three Months Ended | | Six Months Ended | ||||||||
| | June 30, | | June 30, | ||||||||
|
| 2023 |
| 2022 |
| 2023 |
| 2022 | ||||
Research and development expense | | $ | 549 | | $ | 637 | | $ | 1,122 | | $ | 1,233 |
General and administrative expense | |
| 1,251 | |
| 2,072 | |
| 2,469 | |
| 3,505 |
Total stock-based compensation expense | | $ | 1,800 | | $ | 2,709 | | $ | 3,591 | | $ | 4,738 |
| 1 Unleashing the Potential of Immuno-Oncology Therapies August 14, 2023 © 2023 Xilio Therapeutics, Inc. |
| 2 Forward-Looking Statements and Disclaimers This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding plans, timing and expectations related to: reporting preliminary data from the Phase 1/2 clinical trial for XTX202, including the anticipated number of patients treated at the 1 mg/kg dose level or higher; evaluating XTX202 at a second dose level in the Phase 2 clinical trial; reporting preliminary safety data from the Phase 1 clinical trial for XTX301; activating clinical trial sites for Phase 1 combination dose escalation portion of the clinical trial evaluating XTX101 in combination with atezolizumab in patients with advanced solid tumors; the potential benefits of any of Xilio’s current or future product candidates in treating patients; Xilio’s ability to obtain and maintain sufficient cash resources to fund its operations beyond the end of the second quarter of 2024; and Xilio’s strategy, goals and anticipated financial performance, milestones, business plans and focus. The words “aim,” “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “seek,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of important risks, uncertainties and other factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation, including, without limitation, risks and uncertainties related to ongoing and planned research and development activities, including initiating, conducting or completing preclinical studies and clinical trials and the timing and results of such preclinical studies or clinical trials; the delay of any current or planned preclinical studies or clinical trials or the development of Xilio’s current or future product candidates; Xilio’s ability to obtain and maintain sufficient preclinical and clinical supply of current or future product candidates; or Xilio’s advancement of multiple early-stage programs. There can be no assurance that interim or preliminary preclinical or clinical data or results will be predictive of future preclinical or clinical data or results, including, without limitation, the preliminary intra-tumoral pharmacodynamic data reported for two patients treated with XTX202 who each had an optional on-treatment tumor biopsy; Xilio’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; results from preclinical studies or clinical trials for Xilio’s product candidates, which may not support further development of such product candidates; actions of regulatory agencies, which may affect the initiation, timing and progress of current or future clinical trials; Xilio’s ability to obtain, maintain and enforce patent and other intellectual property protection for current or future product candidates; Xilio’s ability to obtain and maintain sufficient cash resources to fund its operations beyond the end of the second quarter of 2024; the impact of international trade policies on Xilio’s business, including U.S. and China trade policies; and Xilio’s ability to maintain its clinical trial collaboration with Roche to develop XTX101 in combination with atezolizumab. These and other risks and uncertainties are described in greater detail in the section entitled “Risk Factors” in Xilio’s filings with the U.S. Securities and Exchange Commission (SEC), including Xilio’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, as well as other subsequent filings that Xilio has made or may make with the SEC in the future. Any forward-looking statements contained in this presentation represent Xilio’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Xilio explicitly disclaims any obligation to update any forward-looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Xilio’s own internal estimates and research. While Xilio believes these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, Xilio has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Tecentriq is a registered trademark of Genentech USA Inc., a member of the Roche Group. |
| 3 Immuno-Oncology Therapy has Curative Potential but is Often Limited by Systemic Toxicity • Immuno-oncology (IO) therapies have transformed the treatment landscape and long-term outlook for some patients with advanced cancer • Treatment potential for some of the most exciting IO targets has been impeded by dose-limiting systemic toxicity Patient Portrayal The Critical Challenge: Maximizing Efficacy While Improving Tolerability Xilio (ex-il-ee-oh) believes the next revolution in IO cancer therapies will trick tumors into activating their own treatments, while simultaneously sparing healthy tissues and cells |
| 4 Xilio’s Novel Tumor-Activated Molecules Are Designed to Overcome the Limitations of Systemically Active Treatments Cleavage Site Half-Life Extension Domain Effector Domain Masking Domain Antibody Example Cytokine Example • Harness and focus the power of the immune system to fight cancer • Novel design to outsmart tumors – using tumor growth activity against itself - Tumor proteases activate a switch in molecules to unleash active agent inside tumor microenvironment (TME) • Each molecule custom-designed using our proprietary geographically precise solutions (GPS) platform for tumor-selectivity with a masking domain that is designed to prevent interaction with healthy tissue and cells - Molecules are activated by tumor’s dysregulated matrix metalloproteases (MMPs) mAb: monoclonal antibody Masking Domain Variable Domain Variable Domain Masking Domain Cleavage Sites |
| 5 Xilio’s Molecules Are Designed to Be Selectively Activated in the TME by MMPs In vitro recombinant MMP kinetic cleavage assay Tumor Plasma Spleen Liver Kidney Lung 0 2 4 6 Fold Difference in % Active Drug in Tumor or Normal Tissues vs Plasma (Avg) Tumor-specific activation +MMPs No MMP 0 500 1000 1500 2000 2500 0 20 40 60 80 100 Time (min) Product (%) Demonstrated in >400 patient specimens Left panel: Time-course of XTX301 activation by recombinant human MMPs. Middle panel: Mice bearing MC38 syngeneic colorectal carcinoma tumors were dosed with mXTX301 (murine surrogate for XTX301), and the percent activated molecule was measured 72h post dose in tumor, plasma, spleen, liver, kidney and lung. Average % active molecule in plasma was set to 1 and fold difference in average % active drug in tumor or normal tissues vs plasma is shown. Right panel: Activation of XTX101, XTX202 or XTX301 assessed in tumor biopsies ex vivo. High activation efficiency across human solid tumors ex vivo Tumor-specific activation in vivo High activation efficiency across human solid tumors ex vivo Tumor Type Confirmed High Activation Efficiency (<24 hours) XTX101 (anti-CTLA-4) XTX202 (IL-2) XTX301 (IL-12) Colon H&N Prostate RCC Lung Melanoma Plasma |
| 6 Program Initial Tumor Types Mechanism of Action Discovery IND-Enabling Phase 1 Phase 2 Phase 3 Antibody Program XTX101 (1) Advanced Solid Tumors and MSS CRC Anti-CTLA-4 Cytokine Programs XTX202 (2) Advanced RCC and Melanoma IL-2 XTX301 (3) Advanced Solid Tumors IL-12 Multifunctional Program Multifunctional Advanced Solid Tumors PD-1/IL-2 1. Xilio plans to evaluate XTX101 in combination with atezolizumab (Tecentriq®) in a Phase 1/2 clinical trial under a clinical trial collaboration with Roche. The Phase 1 portion is designed to assess the safety and tolerability of the combination in dose escalation in patients with advanced solid tumors, and the planned Phase 2 portion is designed to assess the safety and efficacy of the combination in patients with MSS CRC. 2. Initially evaluating XTX202 as a monotherapy in patients with unresectable or metastatic melanoma and metastatic RCC prior to evaluating XTX202 in combination with an anti-PD-1/PD-L1 for the treatment of patients with NSCLC or potential expansion into additional cancer indications as a monotherapy or combination therapy. 3. Initially plan to evaluate XTX301 as a monotherapy for the treatment of advanced solid tumors. CRC: colorectal cancer; MSS: microsatellite stable; NSCLC: non-small cell lung cancer; RCC: renal cell carcinoma. Building a Pipeline of Novel, Tumor-Activated Immuno-Oncology Therapies |
| 7 CTLA-4 Evolving Paradigm and Opportunity for a Tumor-Activated Anti-CTLA-4 |
| Trial conducted by Bristol Myers Squibb. 8 Ascierto et al., J Immunother Cancer (2020); Larkin et al., N Engl J Med (2015); Wolchok et al., Lancet (2010); Hamid et al., J Transl Med (2011); Lebbe et al., J Clin Oncol (2019); Weber et al., J Clin Oncol (2012). AE: adverse event; irAE: immune-related adverse event; OS: overall survival. Ipilimumab Data Demonstrated Transformative Potential of High Dose Anti-CTLA-4 Ipilimumab (10 mg/kg; USPI N=471) irAE All Grades G3+ Gastrointestinal 31% (up to 39% in Phase 2) 16% Endocrine 28% 9% Skin 25% (up to 47% in Phase 2) 4% Liver 15% 11% Infusion Related 3% Dose (mg/kg) Median OS Grade 3/4 irAEs Discontinuations Comments 3 11.5 Months 14% 19% • Standard approved dose 10 15.7 Months 30% 31% • Increased OS indicates greater efficacy • Increased irAEs indicate greater toxicity Time (Weeks) Toxicity Grade High-Dose Ipilimumab Improved Efficacy, But Limited by Toxicity Improved efficacy seen with 10 mg/kg dose, but greater toxicity limits clinical use to 3 mg/kg dose Ipilimumab dose further reduced in combination with PD-(L)1, typically to 1 mg/kg 3-fold increase in therapeutic index has significant potential for transformational outcome 3x Average time to onset of AEs associated with Ipilimumab |
| 9 CTLA-4’s Changing Paradigm: Fc Enhancement to Drive ADCC and High Dose Improves Outcomes 1. Bullock AJ, Grossman JE, Fakih MG, et al: ESMO World Congress on Gastrointestinal Cancer 2022. Abstract LBA-09. Presented June 29, 2022. 2. Phase 1 data reported by Agenus Inc. on January 21, 2023, at ASCO GI Symposium for botensilimab (AGEN1181) in combination with a balstilimab in MSS CRC patients previously treated with chemotherapy and/or with immunotherapy-resistant tumors. 3. Trials conducted by AstraZeneca Pharmaceuticals. Kelley et al., J. Clin. Oncol., 2021; Abou-Alfa et al., J. Clin. Oncol., 2022; Kudo M., Liver Cancer, 2022. Illustration adapted from PDB entry 5TRU; original structure published: Ramagopal et al., Proc Natl Acad Sci 2017 ADCC: antibody-dependent cell-mediated cytotoxicity; ORR: objective response rate; TRAE: treatment-related adverse event; TREG: regulatory T cells; Q4W: once every four weeks. Fc Enhancement to Achieve TREG Depletion • Historically, IO agents have reported 0-5% response rates in MSS CRC (cold tumor): (1) - PD-1 monotherapy: ORR 0% (n=150) - Ipilimumab + nivolumab: ORR 5% (n=20) • Phase 1 data for an Fc-enhanced anti-CTLA-4 in combination with a PD-1 in patients with MSS CRC: (2) - ORR: 23% (n=70) - Phase 1 safety data included any TRAE: Grade 3 (40%) and Grade 4 (3%) CTLA-4 Ipilimumab High Dose CTLA-4 Improved Outcomes • Single high dose (300 mg x1) administration of tremelimumab in combination with an anti-PD-L1 resulted in improved efficacy compared to multiple low doses (75 mg x4 Q4W) (3) |
| 10 XTX101 Tumor-Activated Anti-CTLA-4 |
| 11 Inactive State Active State topspin MMP Activation ADCC-enhanced Fc Variable Domain Variable Domain Activated XTX101 designed to: • bind and block CTLA-4 checkpoint • drive potent ADCC against TREGs that highly express CTLA-4 XTX101: Tumor-Activated, High Affinity Binding, Fc-Enhanced Anti-CTLA-4 Masking Domain Variable Domain Variable Domain Masking Domain Cleavage Sites ADCC-enhanced Fc |
| 12 1. Ipilimumab analog comprising a monoclonal antibody of identical amino acid sequence to ipilimumab that was produced at Xilio for research purposes. CR: complete regression. Left panel: MB49 cells were inoculated subcutaneously into C57BL/6-huCTLA-4 mice. When tumors reached approximately 150 mm3, mice received a single IV dose at the doses indicated in the figure. A two-way ANOVA with Bonferonni’s multiple comparisons post-test was performed to determine the statistical significance of treatment vs. isotype on Day 16 (ns: not significant;*P<0.05; **P<0.01; ***P<0.001;****P<0.0001). Middle panel: MB49 cells were inoculated subcutaneously into C57BL/6-huCTLA4 mice. Mice were dosed 3 mg/kg single-dose i.v. A one-way ANOVA with Bonferroni’s multiple comparisons post-test was performed to determine the statistical significance of treatment vs. isotype control IgG (*P<0.05). Right panel: ADCC experiment utilized reporter gene assay with human FcγRIIIa F158 (low affinity) variant. T cell activation measured in SEB (Staphylococcal enterotoxin b superantigen) assay with test articles at 100 nM concentration. XTX101 Preclinical Profile Differentiated from Ipilimumab (10x Potency, TREG Depletion, Safety) and AGEN1181 (Safety) XTX101 is 10-fold more potent than ipilimumab(1) in in vivo studies XTX101 increased intra-tumoral CD8 T cells and depleted TREGs, while ipilimumab(1) did not XTX101 exhibited enhanced ADCC and T cell activation vs ipilimumab(1) and in line with AGEN1181 XTX101 Ipilimumab(1) IgG Control Ipilimumab* XTX101 0 5 10 15 20 CD8+ T Cells in TME (% CD45+ live cells) * IgG Control Ipilimumab* XTX101 0 5 10 15 20 25 30 Foxp3+CD25+ in TME (% CD4+ live cells) * Tumor CD8 T Cells Tumor TREGs CRs at 0.3 mg/kg IgG Control 0.3 mg/kg XTX101 1.0 mg/kg XTX101 3 mg/kg needed for CRs IgG Control 0.3 mg/kg Ipilimumab* 1.0 mg/kg Ipilimumab* 3.0 mg/kg Ipilimumab* 0.1 1 10 100 1000 10000 100000 0 5000 10000 15000 Concentration (ng/mL) Relative Light Units Ipilimumab* Non-masked XTX101 AGEN1181 IgG Control IgG Non-masked XTX101 AGEN1181 Ipilimumab* 0 5000 10000 15000 20000 25000 IL-2 (pg/mL) T cell Activation (IL-2 Secretion) ADCC Activity |
| 13 Phase 1 Clinical Trial Data for XTX101 |
| 14 Data cutoff date: August 3, 2023. 29 patients have been dosed across all dose levels, including 20 patients dosed in Phase 1A and 9 patients dosed in Phase 1B. 1. Among the 29 patients dosed, data was not available for two patients as of the data cutoff date. 2. Eligible histology includes, but is not limited to, the following: melanoma, squamous cell skin cancer, NSCLC, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, RCC, urothelial carcinoma, MSS instability-high/mismatch repair deficient colorectal or endometrial cancer, cervical cancer, TNBC and mesothelioma. ECOG PS: ECOG performance status; Q6W: once every six weeks; TNBC: triple-negative breast cancer 29 Patients Enrolled in Phase 1 Trial (Phase 1A and 1B) for XTX101 with a Wide Range of Advanced and Treatment Refractory Solid Tumors Phase 1B Monotherapy Expansion PD (2) (n=9 dosed) Phase 1A Monotherapy Dose-Escalation Advanced Solid Tumors (n=20 dosed) Current dose level: 150 mg Q6W Enrollment Completed Ongoing XTX101 Phase 1 Trial Design Patient Characteristics Total (N=27)(1) Demographics Age, median (range) 67 (49, 80) Female 15 (56%) ECOG PS 0 7 (26%) ECOG PS 1 20 (74%) Prior Lines of Anti-Cancer Treatment Median 4 (1-12) 1 2 (7%) 2 4 (15%) 3 6 (22%) 4 7 (26%) 5 3 (11%) 6 and more 5 (19%) Prior Treatment with IO ≥1 12 (44%) Tumor Types Total (N=27)(1) Colorectal 6 NSCLC 4 Pancreatic 3 Squamous cell skin 2 Breast 2 Uterine 2 Merkel cell carcinoma 2 Melanoma 1 Cervical 1 Prostate 1 Gastric 1 Fallopian tube cancer 1 Leiomyosarcoma 1 Treatment Status Total (N=27)(1) Continuing on Treatment 3 Discontinued Treatment 24 Progressive Disease 14 Adverse Events 4 Consent Withdrawal (Hospice) 3 Death Due to Progressive Disease 1 Other 2 |
| 15 150 mg Q6W Identified as RP2D for XTX101 in Phase 1A Data cutoff date: May 2, 2023 AUC: area under the curve; Cmax: maximum concentration; DL: dose level; DLT: dose-limiting toxicity; Q3W: once every three weeks; RP2D: recommended Phase 2 dose. DL5: 150 mg Q6W N=5 DL1: 7 mg Q3W N=2 No DLT DL2: 20 mg Q3W N=1 No DLT DL3: 60 mg Q3W N=6 N=1 Grade 3 Colitis N=2 Grade 3 Colitis DL4: 180 mg Q3W N=6 Target Dose 3X Target Dose RP2D DL5 designed to optimize Cmax from DL4 while maintaining the AUC of DL3 |
| 16 AE Category / Term All TRAEs with ≥10% incidence in any category All Patients at Q3W (7-180 mg) (n=18) RP2D 150 mg Q6W (n=9) Any Grade 3 Any Grade 3 Diarrhea or Colitis 7 (39%) 4 (22%) 1 (11%) 1 (11%) Diarrhea 5 (28%) 1 (6.0) 1 (11%) 1 (11%) (1) Colitis 5 (28%) 4 (22.0) 0 0 Nausea 3 (17%) 0 0 0 Vomiting 3 (17%) 0 0 0 Abdominal pain 2 (11%) 0 0 0 Infusion related reaction (2) 5 (28%) 3 (17%) 0 0 Fatigue 1 (6%) 0 1 (11%) 0 Decreased appetite 1 (6%) 0 1 (11%) 0 Dermatitis 1 (11%) 1 (11%) Dose reduction due to AE 3 1 Treatment discontinuation due to TRAE (3) 4 0 Data cutoff date: August 3, 2023. As of the data cutoff date, safety data were available for 27 patients across all dose levels, including 20 patients dosed in Phase 1A and 7 patients dosed in Phase 1B. 1. Grade 3 diarrhea with onset 10 weeks after the start of treatment (after 2 doses), resolved within 5 days without steroid use, patient tolerated 2 additional XTX101 doses after dose reduction (to 75 mg Q6W) without any symptom recurrence. 2. Infusion related reactions associated with antidrug antibodies (ADA). 3. All treatment discontinuations were due to TRAE for an infusion reaction. 150 mg Q6W Identified as Optimal Dose and Schedule for XTX101 No Grade 4 or 5 AEs Observed at Any Dose Level |
| 17 66% 62% 57% 51% 41% 25% 24% 17% 15% 14% 13% 10% 0% -5% -7% -52% +20% -30% -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 60 70 80 90 100 cSCC: cutaneous squamous cell carcinoma Data cutoff date: August 3, 2023. XTX101 Demonstrated Evidence of Anti-Tumor Activity in Phase 1 Trial (Phase 1A and 1B) Best Percentage Change in Sum of Diameter from Baseline in Target Lesions (%) cSCC NSCLC NSCLC |
| 18 -100 -80 -60 -40 -20 0 20 40 60 80 100 0 5 10 15 20 25 30 35 40 Weeks from First Dose Partial Response Stable Disease +20% -30% New Lesion Data cutoff date: August 3, 2023. As of the data cutoff date, treatment ongoing for one NSCLC patient with confirmed partial response, and all other patients shown in figure have discontinued treatment. XTX101 Demonstrated Prolonged Anti-Tumor Activity in a Patient with PD-L1 Negative NSCLC and Hepatic Metastases Change from Baseline in Target Lesion (%) |
| 19 XTX101 Anti-Tumor Activity in a Patient with PD-L1 Negative NSCLC and Hepatic Metastases • Patient: 66-year-old, female • Diagnosis: Stage 4 NSCLC, PD-L1 negative • Previous Treatment: 1 line of chemotherapy - 4 cycles of paclitaxel and carboplatin - Complete response (CR) - Progressed (four months after CR) • Enrolled in XTX101 trial: Cycle 1 in November 2022 • Dose Level: 150 mg Q6W • Treatment to date: 7 doses of XTX101 administered (continuing on treatment, 36+ weeks) • Related AE: Only Grade 1 fatigue Primary: Lung (NSCLC) Secondary: Liver (Metastatic) Data cutoff date: August 3, 2023. |
| 20 Primary Lung Lesion Decreased in Size and Developed Cavitation on XTX101 Monotherapy Baseline After 9 Weeks of XTX101 After 18 Weeks of XTX101 20 |
| 21 Hepatic Metastases Resolved on XTX101 Monotherapy After 9 weeks of XTX101 Baseline After 18 weeks of XTX101 21 |
| 22 PR: partial response XTX101 Tumor-Selective Activity Demonstrated by Minimal Changes in Peripheral PD Markers in PD-L1 Negative NSCLC Patient with Confirmed PR Fold Change from Baseline in Peripheral PD Markers of Anti-CTLA-4 Activity Pretreatment = baseline On-treatment = cycle 4, day 7 |
| 23 XTX101 Clinical Development Path Pursuing XTX101 in Combination with Atezolizumab in MSS CRC |
| 24 Colorectal Cancer (CRC) – A Growing Threat to Young Adults • In the US, colorectal cancer ranks second in cancer-related deaths overall and is the leading cause in men younger than 50 (1) - Over 150,000 patients diagnosed annually, with ~60% anticipated to have Stage 4 disease at diagnosis (1) - 52,550 CRC deaths projected in 2023, with nearly 4,000 in adults younger than 50 (1) • Majority of patients diagnosed with metastatic disease (~60%) do not have surgery (2) - Primary treatment approach includes chemotherapy and radiation for most patients - Only 2-4% of Stage 4 patients classified as MSI-H are eligible for treatment with immunotherapy, and a subset of these quickly develop immune resistance (3) MSI-H: Microsatellite Instability-High 1. Siegel et al, Colorectal Cancer Statistics, (2023). 2. Cerner Enviza, CancerMPact®Treatment Architecture (2022). 3. Weng et al, Journal of Hematology & Oncology, (2022). 24 |
| 25 Vast Majority of Metastatic Colorectal Cancer is MSS CRC with No Approved IO Treatment Options • MSS CRC represents the vast majority of metastatic CRC (95%) (2) - Characterized by tumors with weak immunogenicity and limited immune cells (making it a “cold tumor”) - Checkpoint inhibitors ineffective in MSS CRC to date - Opportunity exists for IO combinations that together can help mount an adequate immune response • Liver is most common site of metastases in CRC (3) - Over 80% of patients with liver metastases from CRC have unresectable lesions (4) - Long-term survival remains rare, with these patients often excluded from clinical trials, particularly for IO 1. Siegel et al, Colorectal Cancer Statistics, (2023). 2. Ooki et al, Journal of Anus Rectum Colon, (2021). 3. Valderrama-Trevino et al, EJOHG, (2017). 4. Sheth et al, Clinics in Colon and Rectal Surgery (2005). 5. Cerner Enviza, (2023). ~85,000 patients with Stage 4 MSS CRC in the US alone have no IO options available to treat their disease US patients projected to be diagnosed with CRC in 2023 (1) ~150,000 ~60% of patients will be diagnosed with Stage 4 disease (1) ~90,000 ~95% of Stage 4 disease is MSS CRC (2) ~85,000 ~70% of patients of patients with Stage 4 disease develop liver metastases (5) ~60,000 |
| 26 Plan to activate clinical trial sites for combination dose escalation to evaluate XTX101 in combination with atezolizumab in Q4 2023 Data cutoff date: August 3, 2023 1. Eligible histology includes, but is not limited to, the following: melanoma, squamous cell skin cancer, NSCLC, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, RCC, urothelial carcinoma, MSS instability-high/mismatch repair deficient colorectal or endometrial cancer, cervical cancer, TNBC and mesothelioma. 2, Clinical trial research collaboration with Roche to evaluate XTX101 in combination with atezolizumab (Tecentriq®). Clinical Development Plan for XTX101 Phase 1B Monotherapy Expansion PD (1) (n=9 dosed) Current dose level: 150 mg Q6W Enrollment Completed Ongoing Phase 2 XTX101 + atezolizumab in MSS CRC (2) Phase 2 intended to include patients both with and without liver metastases Phase 1A Monotherapy Dose-Escalation Advanced Solid Tumors (n=20 dosed) Phase 1C XTX101 + atezolizumab (2) Combination Dose Escalation Indicates planned future trial |
| 27 Data cutoff date: August 3, 2023. 1. Tumor response was assessed by RECIST version 1.1. XTX101 Clinical Trial Progress and Anticipated Milestones • RP2D defined at 150 mg Q6W with differentiated safety profile • Preliminary PK analyses demonstrated dose-proportional drug exposure and limited active (unmasked) molecule in the periphery • 150 mg dose provided a 2-fold higher Cmax (adjusted for 10x higher potency) compared to ipilimumab 10 mg/kg • Monotherapy confirmed partial response in a patient with PD-L1 negative NSCLC reported at 150 mg Q6W with resolution of hepatic metastases (1) - Tumor-selective activation for XTX101 demonstrated by minimal peripheral PD in this patient • Anticipate activating clinical trial sites for Phase 1 dose escalation evaluating XTX101 in combination with atezolizumab in Q4 2023 Q4 2023 |
| 28 XTX202 Tumor-Activated, Beta-Gamma IL-2 |
| 29 Data represents all patients that received high dose IL-2 (no control group). NR: no response; PFS: progression free survival. High-Dose IL-2 has Curative Potential but Limited by High Systemic Toxicity High-Dose IL-2 • Deep and durable responses • CRs in Melanoma and RCC • High systemic toxicity Low-Dose IL-2 • Tolerable but immunosuppressive The critical challenge in the development of IL-2 therapies is to maximize efficacy while improving patient tolerability High-dose IL-2 resulted in PFS >10 years, mostly in patients who achieved a CR |
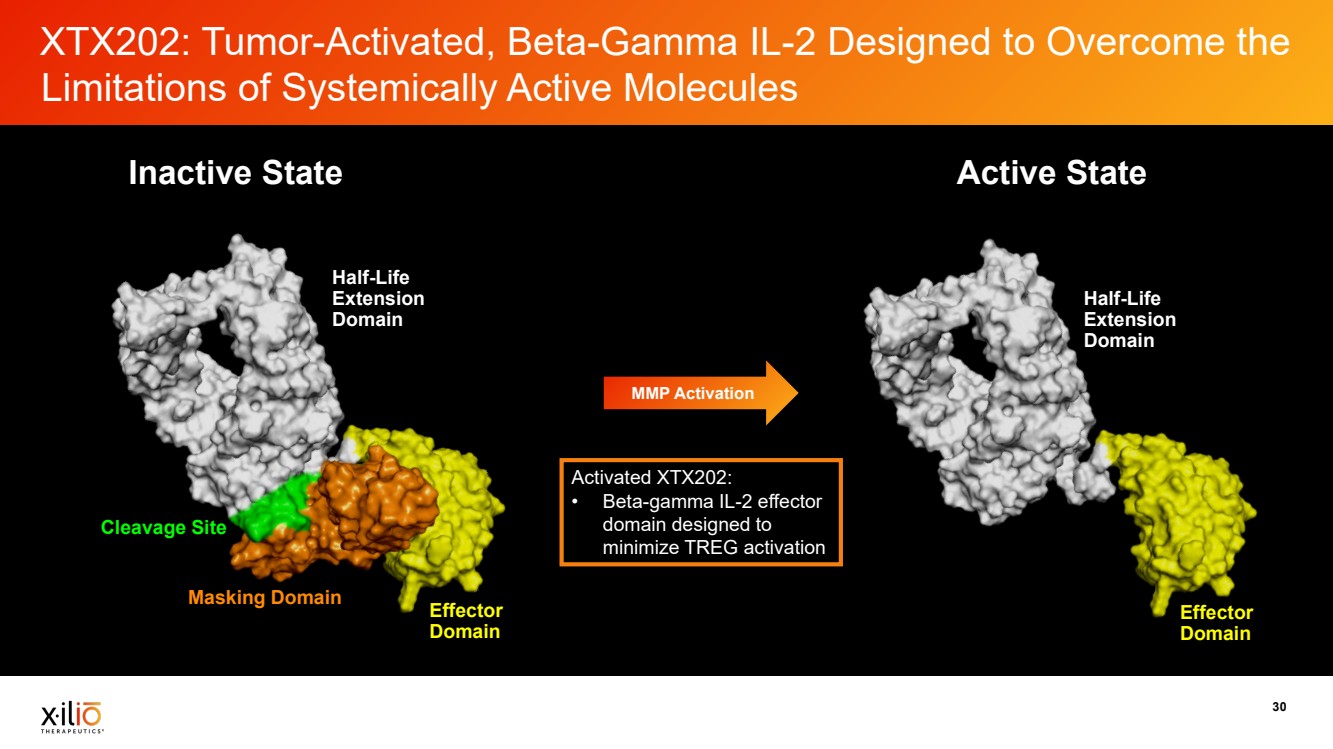
| 30 XTX202: Tumor-Activated, Beta-Gamma IL-2 Designed to Overcome the Limitations of Systemically Active Molecules Cleavage Site Half-Life Extension Domain Effector Domain Masking Domain Half-Life Extension Domain Effector Domain MMP Activation Inactive State Active State Activated XTX202: • Beta-gamma IL-2 effector domain designed to minimize TREG activation |
| MC38 tumor-bearing mice were treated with either vehicle, aldesleukin (3 mg/kg BID) or XTX202 10mg/kg QDx5. Tumor volume was recorded at day 5 post first dose and tumor infiltrating immune cells were phenotyped and 31 enumerated using flow cytometry. One-way ANOVA was performed to determine statistical significance. *p<0.05; **p<0.001. NK: natural killer; TIL: tumor infiltrating lymphocytes. XTX202 Demonstrated TIL Expansion (CD8+ Effector T Cells and NK) and Anti-Tumor Activity Without Significant TREG Stimulation In Vivo XTX202 and aldesleukin featured comparable anti-tumor activity XTX202 and aldesleukin treatment increased CD8+ T cells in tumors XTX202 and aldesleukin treatment increased NK cells in tumors Aldesleukin increased TREGs in tumors while XTX202 had minimal effects Tumor Volume (mm3) Day 5 Tumor CD8+ T Cells Tumor NK Cells Tumor Regulatory T Cells 3.3-fold change |
| 32 Enhancement of In Vivo Activity and Evidence of Memory Response for XTX202 in Combination with Anti-PD-1 0 20 40 60 80 100 0 50 100 Days post-treatment start Probability of Survival Rechallenge ** 0 5 10 15 20 0 500 1000 1500 Days post-treatment start Tumor volume (mm³ + SEM) Naive XTX202 2mg/kg + Anti-mPD-1 0 5 10 15 20 0 500 1000 1500 Days post-treatment start Tumor volume (mm³ + SEM) Vehicle XTX202 2mg/kg Anti-mPD-1 10mg/kg XTX202 2mg/kg + Anti-mPD-1 * ** * TGI (D13) 48% TGI(D13) 69% TGI (D13) 92% Data Presented at Society for Immunotherapy of Cancer (SITC) in November 2022 TGI (D13) 48% TGI (D13) 69% TGI (D13) 92% Anti-tumor activity of XTX202 as a single agent and in combination with anti-mPD-1 was evaluated in hFcRn Tg32 transgenic mice bearing the murine MB49 bladder carcinoma model. The combination of XTX202 with anti-mPD-1 further improved anti-tumor activity with TGI 92% on Day 13 (Data presented as mean ±SEM, two-way ANOVA followed by post hoc Dunnett’s test, *P < 0.05; **P < 0.005). The treatment with XTX202 alone or in combination with anti-mPD-1 improved animal survival from 19 days to 27.5 and 38 days, respectively (Geham-Breslow-Wilcoxon test, **P < 0.01). A mouse with complete regression of MB49 tumor after combination therapy with XTX202 and anti-mPD-1 was resistant to tumor rechallenge with autologous MB49 tumor implanted on the opposite flank. mAb: monoclonal antibody; TGI: tumor growth inhibition. Enhanced in vivo activity observed with combination of XTX202 and anti-PD-1 mAb XTX202 combination with anti-PD-1 induced complete responses in subset of animals Complete responders rejected tumors upon rechallenge, indicating evidence of memory response |
| 33 As of August 14, 2023 Clinical Development Plan for XTX202 Potential to Explore Additional Phase 2 Trials for XTX202 in Combination with an anti-PD-(L)1: Melanoma, RCC, Lung Cancer Indicates potential future cohort or trial Current dose level: 4.0 mg/kg Phase 2A Monotherapy Expansion RCC Cohort Phase 2B Monotherapy Expansion Melanoma Cohort Phase 2C PD-1 Combination Dose Escalation Phase 1B Monotherapy PD Cohort “Hot Tumors” Monotherapy Expansion Potential pivotal trial in RCC and/or melanoma Initial dose level: 1.4 mg/kg Dose level: 2.8 mg/kg Phase 1A Monotherapy Dose Escalation Advanced Solid Tumors |
| Aldesleukin dose if full indicated administration completed (0.037mg/kg*14 over 5 days + 9 days rest + 0.037 mg/kg*14 over 5 days). In preclinical studies, XTX202 at a total dose of 20 mg/kg had similar activity for tumor growth inhibition and 34 PD to aldesleukin at a total dose of 24 mg/kg. DO: dose optimization; DE: dose escalation. XTX202 Has Achieved Dose Levels Beyond High-Dose IL-2 High-Dose IL-2 and IL-2 Clinical Molecules (doses in mg/kg) Aldesleukin at the approved dose is associated with severe systemic toxicity 1.036 0.006 0.024 0.030 0.090 0.364 2.8 Aldesleukin Bempegaldesleukin THOR707 Nemvaleukin MDNA11 ANV419 XTX202 Systemically Active Sytemically Active Systemically Active Systemically Active Systemically Active Systemically Active Tumor-Activated Clinigen Nektar Sanofi Alkermes Medicenna Anaveon Xilio Approved Negative Phase 3 DO RP2D DE DE DE + P2 |
| Mojgan 35 Ahmadzadeh, Steven A. Rosenberg 2006 DOI: 10.1182/blood-2005-06-2399. 2. ASCO 2017, Abstract #2545. 3. ASCO 2022, Abstract # 2500. Results do not represent a head-to-head trial for 3rd party products and XTX101. Peripheral lymphocytosis is a PD marker of IL-2 biology. hr: human recombinant; ALC: absolute lymphocyte count No Peripheral Lymphocytosis Observed with XTX202 in Patients at the 1 mg/kg Dose Level Aldesleukin (1) hr IL-2 First Generation Bempegaldesleukin (2) NKTR-214 Second Generation Nemvaleukin Alfa (3) ALKS-4230 Second Generation Tumor-Activated XTX202 Third Generation Dose: 3 daily doses of high-dose IL-2 (720K IU/kg for 9 doses) Dose: 0.006 mg/kg Dose: 0.006 mg/kg (RP2D) IV x 5D Q3W Dose: 1 mg/kg Q3W Cell Types Immune cell expansion 2000 1500 1000 500 0 Absolute count (cells/µl blood) 0 10 20 30 40 Day XTX202: decrease in ALC count observed on Day 2; magnitude of increase in ALC on Day 7 lower than what has been reported for aldesleukin and bempeg |
| 36 • 3.4 average increase in CD8 cell count observed in tumors treated with XTX202 from baseline • Clinical data consistent with 3.3-fold change observed in preclinical data Patients had an optional on-treatment tumor biopsy and were the only four patients treated with XTX202 for whom a tumor biopsy analysis was available as of August 1, 2023. CD8+ T cells assessed by FACS for peripheral blood and IHC for tumor. Relative fold-change in CD8+ cells in tumor takes into account increase in stromal TILs and CD8+ IHC (%TIL post-treatment x %CD8+ post-treatment over (%TIL pre-treatment x %CD8+ pre-treatment). Tumor-Selective Increases in CD8+ Effector T Cells Observed with XTX202 in Patients Patient #1: Melanoma patient treated with XTX202 at 0.38mg/kg Q2W (dose level 2) Relative Fold-Change in CD8+ T cells Compared to Pre-Treatment Periphery Tumor 0 2 4 6 8 Relative fold change in %CD8 T cells 3.3-fold Increase Patient #2: RCC patient treated with XTX202 at 0.53mg/kg Q2W (dose level 3) Relative Fold-Change in CD8+ T cells Compared to Pre-Treatment Periphery Tumor 0 2 4 6 8 Relative fold change in %CD8 T cells 7-fold Increase Patient #3: RCC patient treated with XTX202 at 1mg/kg Q2W (dose level 4) Relative Fold-Change in CD8+ T cells Compared to Pre-Treatment Periphery Tumor 0 2 4 6 8 Relative fold change in %CD8 T cells 1.16-fold Increase Patient #4: Rectal patient treated with XTX202 at 2.8mg/kg Q2W (dose level 6) Relative Fold-Change in CD8+ T cells Compared to Pre-Treatment Periphery Tumor 0 2 4 6 8 Relative fold change in %CD8 T cells 2.2-fold Increase |
| 37 XTX202 Clinical Trial Progress • Administered as an outpatient regimen • PK supports once every three-week dosing schedule • Surpassed target dose range of 1 mg/kg (dose level four) in Phase 1 monotherapy dose-escalation - No signs or symptoms of VLS observed through 2.8 mg / kg (dose level six) - Currently dosing patients at 4.0 mg/kg (dose level seven) • Tumor-selective increases in CD8+ effector T cells observed in four patients following XTX202 treatment • Patients received up to 17 cycles of treatment to date in Phase 1 monotherapy dose escalation • Phase 2 open and dosing patients at initial RP2D of 1.4 mg/kg - Based on continued Phase 1 dose-escalation, plan to explore opportunities to evaluate XTX202 at an additional, higher dose level in Phase 2 Q4 2023 • Plan to report preliminary anti-tumor activity, PK/PD and safety data in at least 20 evaluable patients treated at dose levels at or above 1 mg/kg in the Phase 1/2 trial in early November 2023 |
| 38 Multiple Combination Opportunities Enabled by XTX202 Properties: Tumor-Activated, Well-Tolerated Preclinically and Clinically-Validated Target • Monoclonal antibodies • (Checkpoint inhibitors; ADCC-inducing mAbs; • T-cell engagers) Other pro-inflammatory cytokines (IL-12; IL-18) Cell therapies (TIL, T-cell receptor (TCR)) XTX202 (IL-2) Cancer vaccines XTX202’s novel proprietary design has potential for numerous combination opportunities |
| 39 XTX301 Tumor -Activated IL -12 |
| 40 • IL-12 has significant potential as a potent IO therapeutic agent in cold tumors • Poor tolerability has limited its clinical progress for decades • No currently approved IL-12 agents INFγ is a pleiotropic molecule with associated antiproliferative, pro-apoptotic and antitumor mechanisms. Th1-type cytokines tend to produce the proinflammatory responses responsible for killing intracellular parasites and for perpetuating autoimmune responses. INFγ: interferon gamma; MTD: maximum tolerated dose; ng/kg: nanograms/kilogram. The Compelling Potential of IL-12 as a Therapeutic Agent IL-12 Has Highly Compelling Biology for IO Applications Exquisitely potent stimulator of NK and T cell cytotoxicity and INFγ production Demonstrated single agent objective responses in patients, but poorly tolerated (MTD <500 ng/kg on repeat dosing) Capable of polarizing CD4 T-cells towards Th1 phenotype, thus driving cellular immunity against infection and cancer Robust INFγ induction results in broad remodeling of the TME towards a more immune-permissive environment |
| 41 Adapted from “Cold vs Hot Tumors”, by BioRender.com, 2022. Retrieved from https://app.biorender.com/biorender-templates Barraondo et al., Clin. Cancer Res., 2018. Nguyen et al., Front. Immunol., 2020. MDSC: myeloid-derived suppressor cells Hot Tumor IL-12 Can Remodel Cold Tumor Microenvironment Towards a Pro-Inflammatory (Hot) State that Favors Anti-Tumor Immunity • Lack of CD8+ T cells and NK cells within tumor • Presence of immune suppressive cells (TREGs, MDSCs) • Poor response to checkpoint inhibitors • CD8+ T cells and NK cells are abundant in tumor • Pro-inflammatory microenvironment • Improved prognosis and effective killing of tumor cells with immunotherapy treatment Cold Tumor IL-12 |
| 42 XTX301: Tumor-Activated IL-12 Designed to Overcome the Limitations of Systemic Recombinant Human IL-12 Inactive State Active State MMP Activation Half-Life Extension Domain Masking Domain Effector Domain Cleavage Site Effector Activated XTX301: Domain • Optimized short half-life IL-12 (half-life extension domain not retained) |
| 43 Tumor Growth Body Weight mXTX301 is a murine surrogate for XTX301. MC38 model: s.c. 0.5x106 cells; single IV dose of mXTX301 and mXTX302 on Day 0. Tumor growth data shown as mean±SEM. Tumor volume data was assessed by a two-way ANOVA followed by Bonferroni post hoc test on Day 11 compared to vehicle treated animals. ****p<0.0001 for all mXTX301 treatment groups. Body weight data are shown as mean ±SEM. A two-way ANOVA followed by Bonferroni post hoc test compared to vehicle treated animals was performed **p<0.005, ****p<0.0001. mXTX301 Demonstrated Dose-Dependent Anti-Tumor Activity Without Body Weight Loss In Vivo …without causing body weight loss High-dose mXTX301 demonstrated significant anti-tumor activity… Data Presented at New York Academy of Sciences’ Frontiers in Cancer Immunotherapy in May 2022 |
| 44 mXTX301 is a murine surrogate for XTX301. Left panel: MC38 tumor bearing mice (n= 5 per group) were treated with a single IV dose of mXTX301 at 0.39 mg/kg or vehicle and immune cells were phenotyped using FACS. The number of cells for each immune phenotype was calculated per g of tissue and the ratio of cells after mXTX301 treatment to after vehicle treatment is presented as mean ± SD. Changes in the ratio of each cell type in spleen and tumor were assessed by an unpaired t test. *P < 0.05, **P < 0.005. Right panel: Tumors from mice treated with vehicle, 0.39 mg/kg mXTX301 were profiled by RNAseq. Left Heatmap: Color tracks with z-score-transformed relative expression of each gene across samples (blue, under-expression compared to the mean; red: over-expression compared to the mean). Right Heatmap: Color shows significance (-log10 Fisher_PVal) of pathway enrichment (rows are pathways or gene-sets). mXTX301 Induced Tumor-Specific Pharmacology In Vivo • • CD45 CD3 CD4 CD8 0 2 4 6 Fold change over Vehicle Tumor Spleen ns ✱✱ ✱ ✱✱ Vehicle mXTX301 Day 4 Day 7 Day 4 Day 7 -2 -1 0 1 2 Gene mXTX301 induced specific T cell recruitment into the tumor with minimal peripheral effects mXTX301 induced pro-inflammatory gene programs and broadly remodeled TME toward inflamed state |
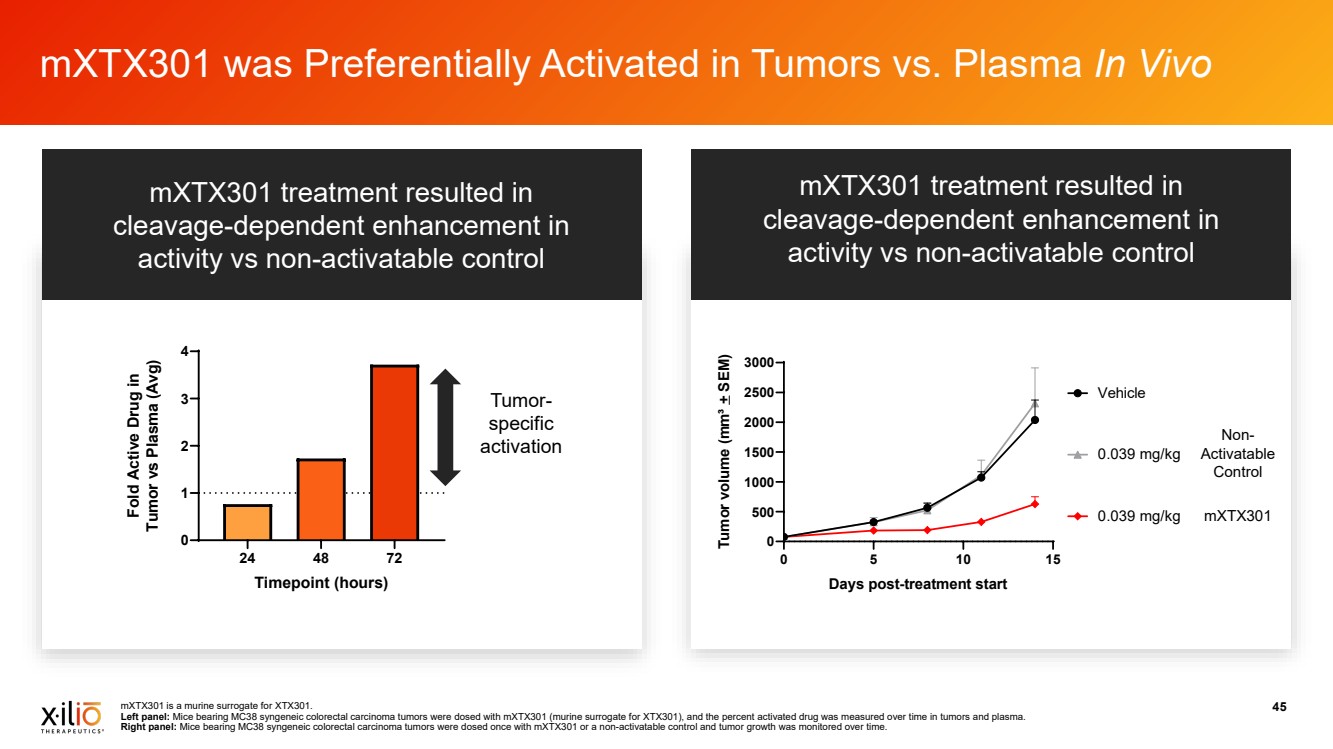
| mXTX301 is a murine surrogate for XTX301. 45 Left panel: Mice bearing MC38 syngeneic colorectal carcinoma tumors were dosed with mXTX301 (murine surrogate for XTX301), and the percent activated drug was measured over time in tumors and plasma. Right panel: Mice bearing MC38 syngeneic colorectal carcinoma tumors were dosed once with mXTX301 or a non-activatable control and tumor growth was monitored over time. mXTX301 was Preferentially Activated in Tumors vs. Plasma In Vivo • • 0 5 10 15 0 500 1000 1500 2000 2500 3000 Days post-treatment start Tumor volume (mm³ + SEM) 0.039 mg/kg Vehicle 0.039 mg/kg Non-Activatable Control mXTX301 24 48 72 0 1 2 3 4 Timepoint (hours) Fold Active Drug in Tumor vs Plasma (Avg) Tumor-specific activation mXTX301 treatment resulted in cleavage-dependent enhancement in activity vs non-activatable control mXTX301 treatment resulted in cleavage-dependent enhancement in activity vs non-activatable control |
| 46 • XTX301 was tolerated at doses up to 2.0 mg/kg Q1W x4 in NHP (HNSTD) • mXTX301 induced tumor regressions in murine model following a single dose of 0.13 mg/kg HNSTD: highest non-severely toxic dose; Q1W: once every week. XTX301 Preclinical Data Support Potential for Broad Therapeutic Index 0 24 48 72 96 120 144 168 1 10 100 1000 10000 Timepoint (hours) Drug Exposure AUC 0-168h (hr* μg/ml) Tolerable exposure in NHP (HNSTD) Exposure range for anti-tumor activity Tumor regressions Tumor growth inhibition Therapeutic index Compound In Vivo Model Dose (mg/kg) AUC0-168 (hr*μg/mL) Estimated Therapeutic Index (AUCSafety / AUCActivity) mXTX301 Anti-tumor activity (murine) 0.13 37.8 67 XTX301 Safety (NHP) 2.0 2540 |
| 47 μg: micrograms. Clinical Development Plans for XTX301 Phase 1A Monotherapy Dose-Escalation Advanced Solid Tumors Phase 1B — 1 Monotherapy PD Cohort “Hot Tumors” Phase 1B — 2 Monotherapy PD Cohort “Cold Tumors” Backfill Cohort Phase 1C Combination with PD-(L)1 Dose-Escalation Advanced Solid Tumors Phase 2 XTX301 Monotherapy Head and Neck Cancer and NSCLC Phase 2 XTX301+PD-(L)1 in MSS CRC or Pancreatic Cancer Initial dose level: 5μg/kg (0.005 mg/kg) Potential to Explore Additional Phase 2 Trials for XTX301 in Combination with an anti-PD-(L)1: NSCLC, Head and Neck, Melanoma, TNBC, MSI-H CRC, Prostate, Ovarian, Pancreas, MSS CRC Indicates potential future cohort or trial |
| 48 XTX301 Progress and Anticipated 2023 Clinical Milestones • Demonstrated dose-dependent anti-tumor activity without significant body weight loss in vivo • Preferentially activated in tumors vs plasma in vivo • Preferentially activated in human patient tumors vs. plasma ex vivo • Phase 1 initiated at starting dose of 5μg/kg (0.005 mg/kg) Q3W - 10x higher than the MTD for recombinant human IL-12 of 0.5 μg/kg (1) • Anticipate reporting preliminary Phase 1 safety data into 3rd dose level in Q4 2023 Q4 2023 1. Portielje et al. Clin Cancer Res. 1999 Dec;5(12):3983-9. |
| 49 Preclinical Proof-of-Concept Human Translational Proof-of-Concept Peripheral Masking In Clinic Tumor-Activation in Clinic Clinical Anti-Tumor Activity XTX101 (Anti-CTLA-4) Phase 1A Complete Phase 1B Dosing XTX202 (IL-2) Phase 1B and Phase 2 Dosing XTX301 (IL-12) Phase 1A Dosing Multifunctional (PD-1/IL-2) Research Program Executing on Our Vision to Deliver Tumor-Activated Immuno-Oncology Therapies Created Through Our Unique and Efficient Design Process Anticipated Early November 2023 Anticipated Q4 2023 |
| 50 Xilio is Positioned for Multiple Anticipated Clinical Milestones XTX101 (Anti-CTLA-4) Reported Clinical Data Preliminary anti-tumor activity, safety and PK/PD data from Phase 1 trial (monotherapy) XTX202 (IL-2) Plan to Report Clinical Data Preliminary anti-tumor activity, safety and PK/PD data in at least 20 evaluable patients from Phase 1/2 trial (monotherapy) XTX301 (IL-12) Plan to Report Clinical Data Preliminary safety data from Phase 1 trial into 3rd dose level (monotherapy dose escalation) Q4 2023 Q2 2023 XTX101 (Anti-CTLA-4) Initiate Combination Dose Escalation Activate clinical trial sites for Phase 1 dose escalation evaluating XTX101 in combination with atezolizumab XTX202 (IL-2) Initiated Clinical Trial Enrollment in Phase 2 trial (monotherapy) XTX101 (Anti-CTLA-4) Announced Clinical Collaboration Advancing Phase 1/2 development of XTX101 in combination with atezolizumab Q3 2023 |
| 51 Anticipate Existing Cash and Cash Equivalents Sufficient to Fund Operating Expenses and Capital Expenditure Requirements Into the End of Q2 2024 Balance Sheet June 30, 2023* December 31, 2022 Cash and Cash Equivalents $75.4M $120.4M * Unaudited Statement of Operations Three Months Ended June 30 2023* 2022* Research & Development Expenses $13.2M $16.2M General & Administrative Expenses $6.9M $8.3M Loss from Operations $(20.1M) $(24.6M) |
| 52 Xilio is working to deliver highly potent, localized immunotherapies in cancer and beyond Xilio Therapeutics is a Differentiated IO Company with a Proprietary Tumor-Activated Platform and the Team to Deliver Patient Portrayal |